Abstract
Background: The use of haploidentical allogeneic hematopoietic stem cell transplantation (haplo-HSCT) has increased owing to therapeutic advances that have mitigated the main barriers such as high incidence of graft-versus-host disease (GVHD) and non-relapse mortality (NRM). This is primarily attributable to the elimination of alloreactive T cells in either the patient or the graft. Such T-cell depletion can be performed in vivo early after T-cell-replete haplo-HSCT using post-transplant cyclophosphamide (PTCy). Alternatively, T-cell-depleted haplo-HSCT can be supplemented with T-lymphocytes that are depleted ex vivo of their alloreactive component in the form of ATIR101 (Kiadis Pharma). Patient exposure to PTCy to eliminate donor alloreactive cells can be performed easily and at low cost but may cause patient toxicity and increase relapse rates, and it requires post-transplant immune suppression. Although ATIR101 requires cell manufacturing and is more expensive, it limits toxicity to the patient, enables haplo-HSCT without the use of immunosuppressants, and may reduce relapse rates. Both strategies are promising, but no attempt has yet been made to compare clinical results in similar patient populations to delineate key features of alloreactive T-cell depletion performed either ex vivo or in vivo.
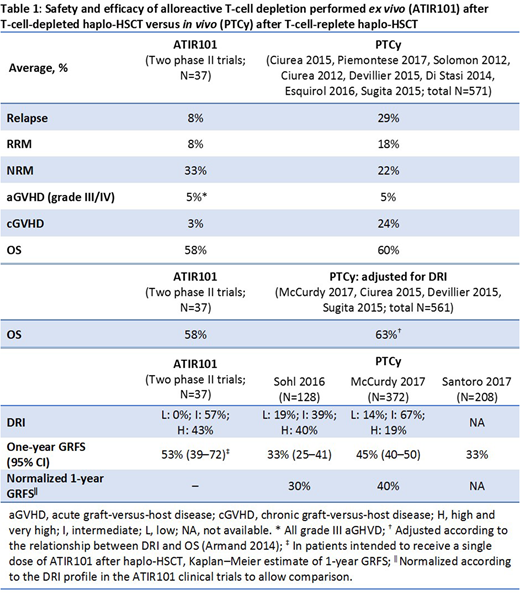
Methods: Data from published retrospective studies (single-site or registry data) were analyzed to assess clinical outcomes of haplo-HSCT plus PTCy. The 1-year outcomes from these studies were compared with results from a pooled analysis of 2 phase II clinical trials of a single dose of ATIR101 (N=37, all patients with AML/MDS/ALL [CR-AIR-007, CR-AIR-008]). Studies in which PTCy was used in patient populations with >50% AML/MDS/ALL were identified (Ciurea 2015, Piemontese 2017, Solomon 2012, Ciurea 2012, Devillier 2015, Di Stasi 2014, Esquirol 2016, Sugita 2015). The 1-year rates of relapse, relapse-related mortality (RRM), NRM, GVHD, and overall survival (OS) for the ATIR101 clinical trials were compared with the weighted average of these outcomes for the identified studies. OS is known to correlate with disease risk index (DRI; Armand 2014); therefore, publications reporting both OS and DRI (McCurdy 2017, Ciurea 2015, Devillier 2015, Sugita 2015) were identified to compare OS. Differences in DRI between PTCy and ATIR101 study populations were adjusted according to the relationship between DRI and OS. Finally, PTCy studies reporting GVHD-free and relapse-free survival (GRFS) were identified (Solh 2016, McCurdy 2017, Santoro 2017). There is a clinically relevant and statistically significant correlation between GRFS and DRI, so 1-year GRFS rates from the 2 studies reporting DRI status (Solh 2016, McCurdy 2017) were also normalized according to the DRI profile in the ATIR101 clinical trials to allow comparison.
Results: The weighted average of PTCy (N=571) outcomes in populations with >50% AML/MDS/ALL vs ATIR101 patient outcomes were: 29% vs 8% for relapse; 18% vs 8% for RRM; 22% vs 33% for NRM; 5% vs 5% for acute GVHD grade III/IV; 24% vs 3% for chronic GVHD; and 60% vs 58% for OS. The OS in DRI-adjusted studies for PTCy (N=561) was similar to that in ATIR101 clinical trials (63% vs 58%, respectively). The GRFS-reporting studies included a total of 708 patients (Sohl 2016, N=128; McCurdy 2017, N=372; Santoro 2017, N=208); 1-year GRFS rates for PTCy in these studies were 33% (95% CI: 25-41), 45% (95% CI: 40-50), and 33% (average), respectively. In the 2 studies reporting DRI (N=500), the DRI profile was more favorable than in the ATIR101 studies and the 1-year GRFS rates normalized in line with the ATIR101 studies were reduced to 30% (Sohl 2016) and 40% (McCurdy 2017). In patients intended to receive a single dose of ATIR101 after haplo-HSCT, Kaplan-Meier estimate of 1-year GRFS was 53% (95% CI 39-72) (Table 1).
Conclusion: This is not a head-to-head comparison, so data should be interpreted with caution. However, in these cross-study analyses, first insights into a potential advantage of ex vivo (ATIR101) over in vivo (PTCy) depletion of alloreactive T cells is suggested, including but not limited to rates of relapse, chronic GVHD, and GRFS. A large, phase III, randomized control trial is thus underway to assess the relative safety and efficacy of ATIR101 after T-cell-depleted haplo-HSCT versus PTCy after T-cell-replete haplo-HSCT (CR-AIR-009 HATCY; NCT02999854).
Devine:Kiadis Pharma: Consultancy. Mielke:Kiadis Pharma: Other: Travel grants, Research Funding. Tuk:Kiadis Pharma: Consultancy. Meewisse:Kiadis Pharma: Employment. Sandler:Kiadis Pharma: Employment. Roy:University of Montreal: Patents & Royalties: Author on patent; Kiadis Pharma: Other: Travel support; Hopital Maisonneuve Rosemont: Patents & Royalties: Author on patent.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal